Llama Nanobodies Are Used To Create Targeted Drug Delivery Systems

Johns Hopkins Medicine researchers claim to have designed tiny drug proteins called nanobodies derived from llama antibodies that could potentially be used to deliver targeted medicines to human muscle cells in “proof of concept” experiments with mouse and human cells and tissues.
The ability to more precisely target such tissues, according to the researchers, could advance the search for safer, more efficient ways to alleviate pain during surgery, treat irregular heart rhythms, and control seizures. Scientists are unsure why they exist only in certain species, such as camelids and sharks, but since their discovery in the 1980s, researchers have studied them for use as a research tool and anti-cancer drug delivery system, with mixed results.
Knowing about such experiments, Johns Hopkins researchers suspected that nanobodies could be useful as a tool for attaching to a cell’s sodium ion channels, which act as a kind of switch that can conduct chemical signals that turn on or off muscle cells.There are nine different types of these switches in the human body, each one specific to a type of tissue such as muscle or nerve.
Because the channel proteins differ only slightly, most medications cannot distinguish between them, posing a safety risk when combined with drugs such as anesthetics. Existing medications, according to the researchers, block pain and sedate patients by “turning off” sodium ion channels in nerves and skeletal muscle, but they can also dangerously lower heart rates and interfere with heart rhythms.
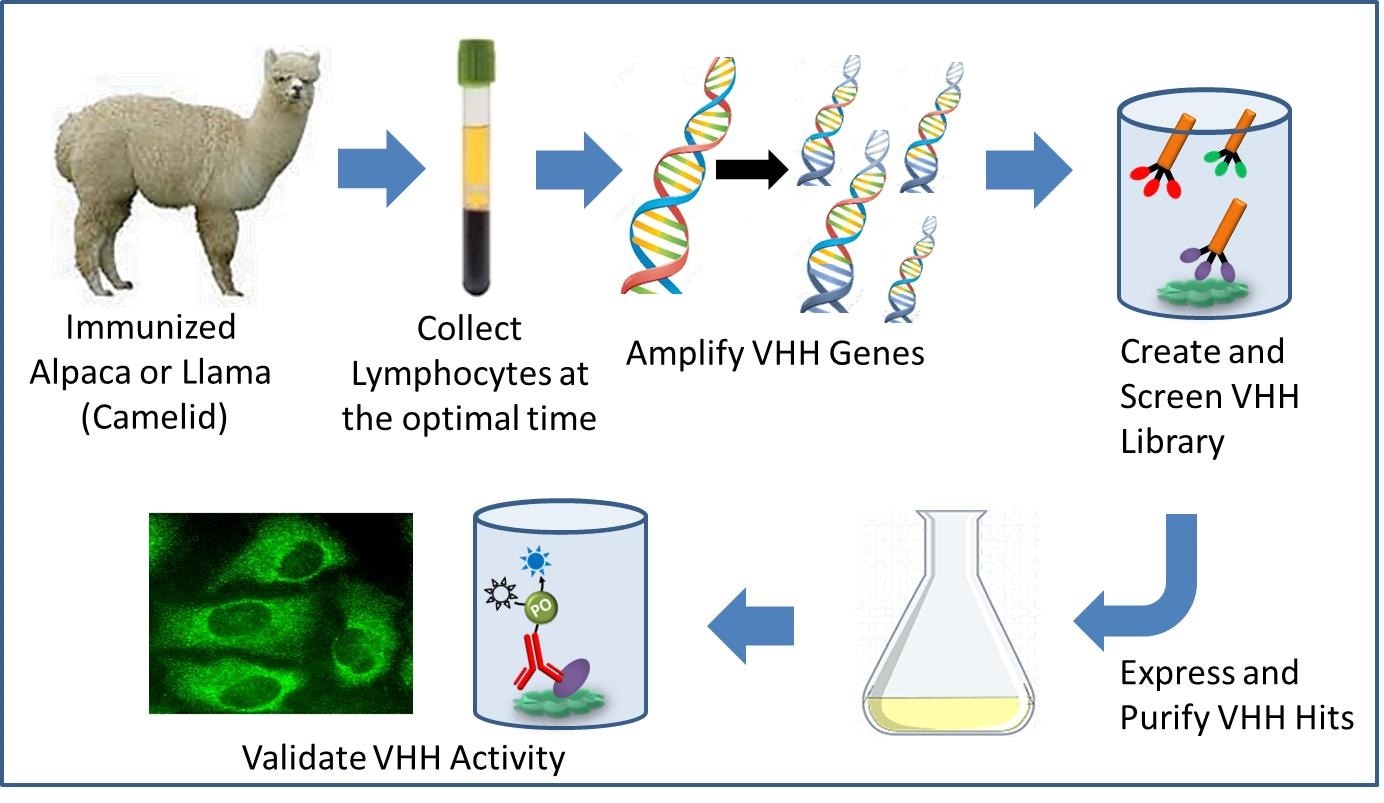
Other studies, according to the Johns Hopkins Medicine researchers, have shown that nanobodies can carry a cargo, a capability that could advance efforts to deliver medications to specific sodium ion channels, eliminating such side effects.” This is why clinicians and pharmaceutical companies are interested in finding drugs that can modulate these channels distinctly — either to turn on or off,” says Sandra Gabelli, Ph.D., associate professor of medicine at Johns Hopkins University. Gabelli recognized that the small size of nanobodies might allow them to bind to areas that larger molecules, such as antibodies, would find difficult to access.
Gabelli’s research team screened a very large library of 10 million nanobodies in proof of concept experiments to develop them as protein biologics that could potentially differentiate between sodium ion channels in muscles and those in nerves.The researchers, working with Columbia University’s Manu Ben-Johny, attached a fluorescent “reporter” molecule to the nanobodies, which light up when it interacts with the sodium channel.
The researchers discovered that two nanobodies, Nb17 and Nb82, were attached to sodium ion channels specific to skeletal muscle and heart muscle by monitoring the glow.The researchers also examined the nanobodies’ stability at various temperatures, which is critical in the development and delivery of drugs to clinics. The researchers discovered that nanobodies Nb17 and Nb82 were resistant to temperatures as high as 168.8 degrees Fahrenheit and 150.8 degrees Fahrenheit, respectively, indicating that these nanobodies would be shelf-stable under normal conditions.The researchers intend to image the nanobody and sodium ion channels bound together next in order to learn more about how this interaction works.








